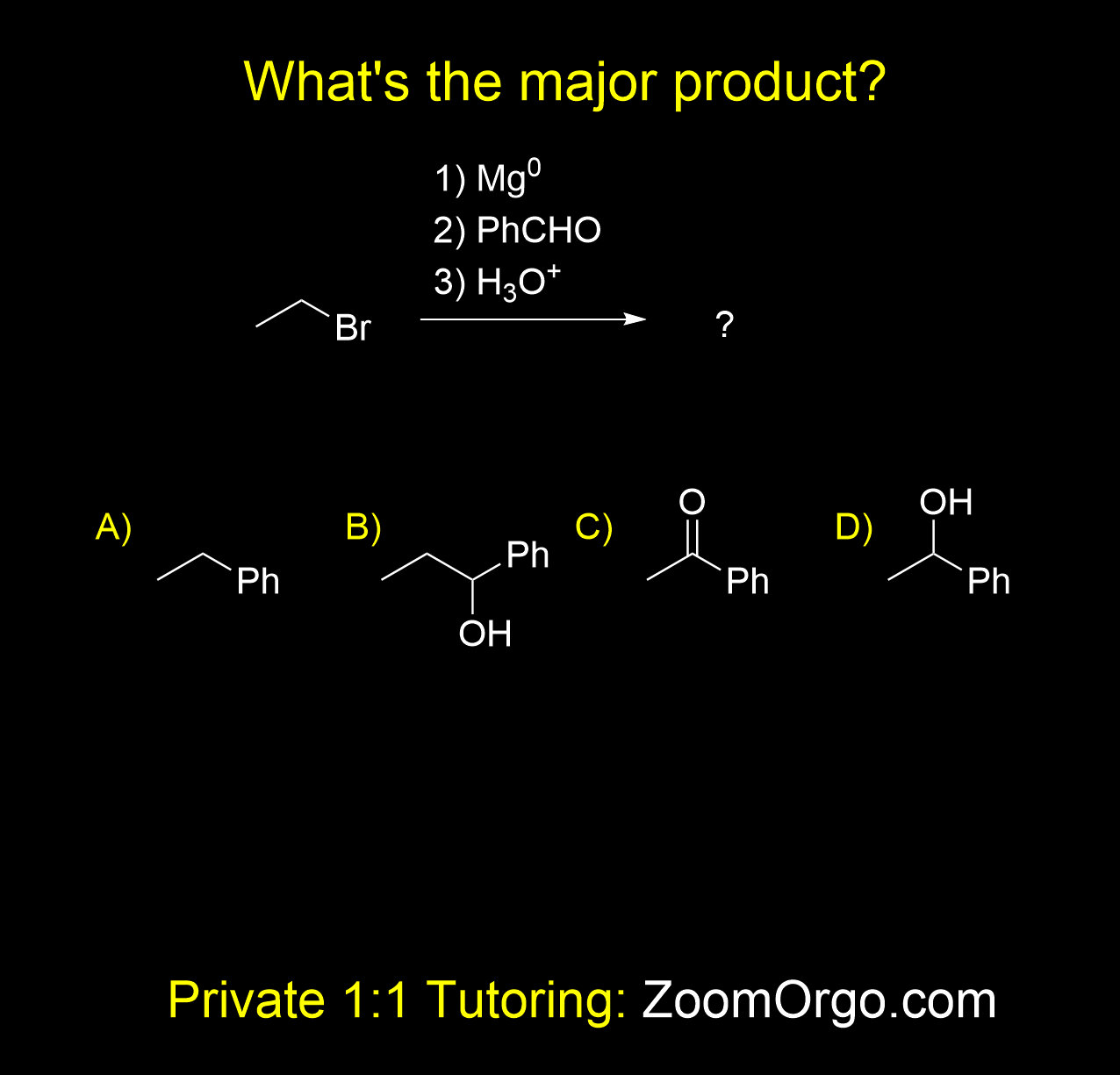
Grignard Addition to Aldehydes
You will often encounter this type of problem in organic chemistry II (or maybe even ochem 1). What happens when a Grignard reagent, made from an alkyl halide + Mg, reacts with an aldehyde? When an alkyl bromide (like bromoethane) is treated with magnesium metal in an aprotic solvent, it forms a Grignard reagent (an organomagnesium halide, e.g. EtMgBr). Grignard reagents are strong nucleophiles and strong bases — they attack electrophilic carbonyl carbons (aldehydes and ketones). After nucleophilic addition, an acidic workup (H₃O⁺) protonates the oxyanion to give an alcohol. In this case, the correct answer is B.